
TOPIC 7: FORMULA BONDING AND NOMENCLATURE | CHEMISTRY FORM 2
The Concept of Valence
Explain the concept of valence
Valency is the capacity of an atom to combine with one or more atoms to form a molecule or compound. Valency also refers the number of electrons that an atom can gain, lose or share in forming a chemical bond with another atom.
The valency (or combining power) depends on the number of electrons in the outermost orbit (or valency shell) involved in the formation of a chemical bond.
The number of electrons in the valency shell is never greater than 7. The outermost electronic configuration is responsible for the variability of the valency.
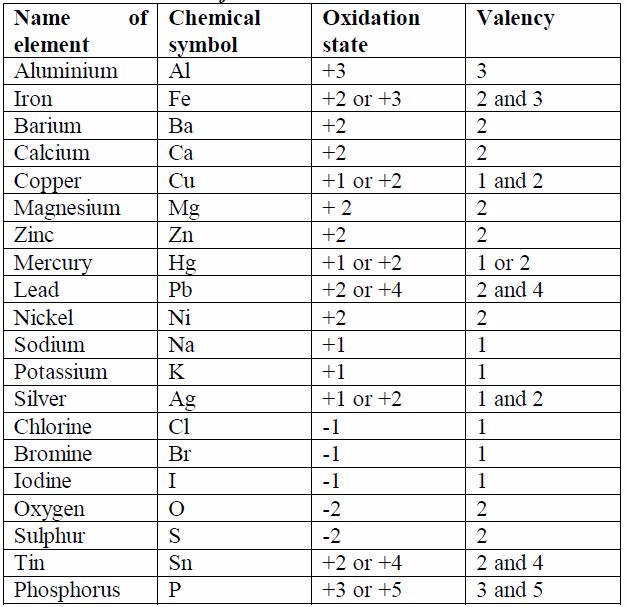
Some elements exhibit more than one valency, i.e., they have variable valencies. Examples of elements with variable valencies are iron (2 and 3), tin (2 and 4) and copper (1 and 2). The other elements with variable valencies are as shown in table 7.1.
Valency and Oxidation States
There is a strong correlation between valency and oxidation state. The
its ion when an element ionizes in solution. An example of this relation
is iron (II) whose oxidation state (or oxidation number) is 2 and its
valency is 2. The same case applies to iron (III).
Other elements with variable valencies such as copper (I) and copper (II) have oxidation state equal to 1 and 2 respectively. The list continues. You will learn more about oxidation states later.
The valencies of the common transition elements should be memorized.
Valencies of the normal elements may be deduced from the group number
they occupy in the Periodic Table. The valencies of elements of group I
to IV are equal to the group numbers they occupy in the periodic table.
The valency of an element in group V to VIII is equal to eight minus the
group number. For example, the valency of chlorine which is in group
VII is 1, i.e. (8 -7) =1. The valency of oxygen in group VI is 2, i.e.
(8-6) =2. Elements in group 0 (or VIII) have zero valency i.e. (8 – 8) =
0.
Simple Formulae of Binary Compounds
Write simple formulae of binary compounds
Chemical formula is a method of representing the molecule of a compound
by using chemical symbols. It is a way of expressing information about
the atoms that constitute a particular chemical compound. The chemical
formula identifies each constituent element by its chemical symbol and
indicates the number of atoms of each element found in each single
molecule of that compound.
symbol for hydrogen atom is H. When two hydrogen atoms join together
they form a molecule,H2. The number 2 to the right and below the symbol
shows the number of atoms a molecule contains. P4 and S8 represents 4
atoms of phosphorus and 8 atoms of sulphur contained in one molecule of
phosphorus and one molecule of sulphur respectively.
the formula for chlorine molecule isCl2, it cannot be expressed as 2Cl.
This is because 2Cl means two atoms of chlorine and not a molecule of
chlorine.H 2 O stands for a molecule of water which consists of two
hydrogen atoms and one oxygen atom.
for a molecule of sulphuric acid containing 2atoms of hydrogen, 1 atom
of sulphur and 4 atoms of oxygen.CaCO3 stands for a molecule of calcium
carbonate containing 1atom of calcium, 1 atom of carbon and 3 atoms of
oxygen.Where it deems necessary to show the number of molecules a
compound contains, this is achieved by writing the appropriate number
before the formula of the compound. A few examples are shown below:
- 2H 2O means two molecules of water
- 3H2 SO4 means three molecules of sulphuric acid
- 5CaCO3 means five molecules of calcium carbonate
It
is important to note that the figure appearing before the formula
multiplies the whole of it. For example, 3H2SO4 stands for 3 molecules
of sulphuric acid containing six atoms of hydrogen, three atoms of
sulphur and twelve atoms of oxygen.It is a big mistake to think that the
number 3 before the molecule multiplies only the symbol which
immediately follows it (that is,H2). This is quite wrong. The 3
multiplies the whole of the formula
binary compound is a compound made of only two types of the reacting
species, for example, sodium chloride (NaCl),which is made of only
sodium and chlorine, is a binary compound. Look at table 7.1. The size
of the charge on an ion isa measure of its valency or combining power.
You will notice that, ignoring the signs for the charge on ions, the
value of the charge on ion is equal to the valency of the atom. You will
need to memorise the valencies of these metals as much as possible so
as to be able to write the formulae of their compounds correctly.
- Metals (or their positively charged ions) must start in theformula, followed by non-metals (or their negatively chargedions).
- Where the formula is to include a radical, the radical must betreated as a single atom and must be bracketed if need be.
- Ammonium ion is to be treated as if it were a metal.
- Positive charges must be equal to negative charges for aneutral molecule or compound.
- Single elements; say Na, K, Si, Ag, etc. should not bebracketed.
- Valencies of metals (or positive ions) should be exchangedand written as subscripts.
- The valency of 1 is simply assumed and not written in theformula.
This
is best shown by using some examples. The following procedure must be
followed when writing the formulae of binary compounds:
Write down correct symbols for atoms of elements or ions that make up the compound.
Write down the valencies of the atoms of the elements.
Interchange
the valencies and write them as subscripts in the final formula of the
compound. Remember that the valency of 1 is not expressed in the
formula. At this final step, the radicals must be bracketed if
necessary.
Write the formula for aluminium oxide:

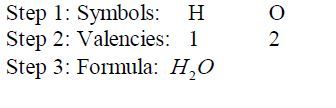
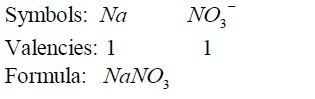

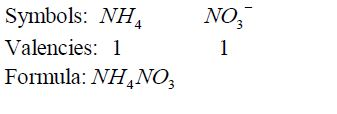
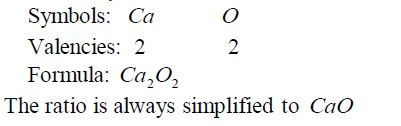
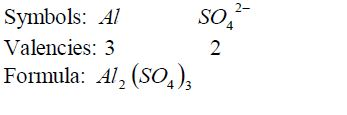
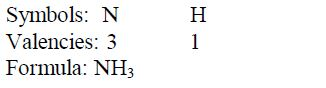
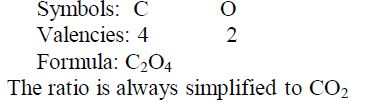
Nomenclature of Inorganic Compounds
term “nomenclature” refers to “system of naming”. The system of naming
in use is that recommended by the IUPAC (International Union of Pure and
Applied Chemistry). The modern system of naming reveals the type of
elements present in a given compound. The old or trivial names have been
dropped out.
common and important compounds have historical names that do not seem
to fit in the system, for example water H O 2 ,ammonia 〈NH3〉, methane ,
CH4and mineral acids such as sulphuric (VI) acid〈H2SO4〉, nitric (V)
acid〈HNO3〉and hydrochloric acid〈HCl〉. Also organic acids such as
ethanoic acid (CH3COOH) are also included in this group. These names are
trivial but they have been adopted in modern nomenclature.If these
exceptions are omitted, there are basic generalizations that are useful:
- If
there is a metal in the compound, it must be named first. In this case
ammonium ion, NH4¹, is regarded as if it were a metal in the compounds
it occurs such as NH4 NO3 , NH Cl 4 , etc. - For elements with
variable valencies such as iron and lead,Roman numerals are included in
the name to indicate the valency of the metal or the ion which is
present. For example,iron (III) chloride contains Fe3+ while iron (II)
chloride contains Fe² . The same case applies to lead (II) and lead
(IV)compounds and so on. - Compounds containing two elements only
(binary compounds) have names ending in …..ide; for example sodium
chloride (NaCl), calcium bromide (CaBr2), magnesium nitrite( Mg3 N2) ,
etc. The important exception to this is hydroxides,which contains the
hydroxide (OH) ion. - Compounds containing a poly atomic ion
(usually containing oxygen) have names that end with …ate; for example
calcium carbonate (CaCO3) , potassium nitrate(KNO3) , magnesium
sulphate(MgSO4) , sodium ethanoate (CH3 COONa) , etc. - The names
of some compounds use prefixes to tell you the number of that particular
atom in the molecule. This is useful if two elements form more than one
compound. For example:carbon monoxide (CO), carbon dioxide (CO2),
nitrogen dioxide NO2 dinitrogen tetra oxide N2 O4 , sulphur dioxide SO2
sulphur trioxide SO3 , etc.
following prefixes indicate the number of atoms in caseslike this: mono
– one; di – two; tri – three; tetra – four; pent –five; hex – six; hept
– seven; oct – eight; non – nine; and dec –ten.
empirical formula is the simplest formula of any compound.It expresses
the simplest ratio of all the atoms or ions that makeup a certain
compound. For example, the empirical formula ofthe compound with the
formula, C2H4is CH2. This means thatthe simplest ratio of (C:H) is 1:2.
This ratio also indicates theratio in which carbon and hydrogen atoms
combine to form thecompound C2H4.
molecular formula is the formula which shows the actual number of all
atoms present in a given compound. For example,the molecular formula of
the above compound is C2H4. This means that two atoms of carbon and four
atoms of hydrogen form the compound. Likewise, the molecular formula of
water isH2O meaning that the compound is made up of two atoms of
hydrogen and one atom of oxygen.
of a compound is the simplest formula which shows its composition by
mass, and which shows the ratio of the number of the different atoms
present in the molecule.
empirical formula differs from the molecular formula of the same
compound since only the molecular formula agrees with the molar mass of
the compound.
- 2 hydrogen atoms combine with 1 oxygen atom to form one molecule of water.
- moles
of hydrogen atoms combine with 1 mole of oxygen atoms. Moles can be
changed to grams using relative atomic masses (RAMs). So we can write: - grams of hydrogen combines with 16 grams of oxygen. In the same way:
- 1g of hydrogen combines with 8g of oxygen.
- 4g of hydrogen combines with 32 of oxygen.
masses of each substance taking part in the reaction are always in the
same ratio.Therefore, from the molecular formula of a compound you can
tell:
- how many moles of different atoms combine;
- how many grams of the different elements combine;
- the number of each kind of atoms of different elements that combine to make up a compound; and
- the percentage of each atom in a compound based on RAMs of each atom And from the empirical formula you can tell:
- the simplest ratio or proportion of the different atoms that combine to form a compound.
empirical formula of ethane C2H4andpropene C3H6with molar masses 28.0g
and 42.0grespectively is CH2(i.e. the same) although the two compounds
possess different molecular formulae and masses.
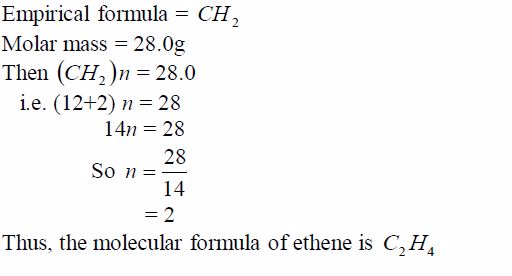
general, the empirical formula multiplied by a whole number,n, gives
the molar mass of the compound. So long as the value of n is known, then
the molecular mass can be deduced.For example, suppose the molecular
mass of ethene is 28.0g, its molecular formula can be deduced thus:
suppose carbon dioxide has a molar mass of 44g and its empirical
formula is CO2. Its molecular formula can be determined thus:

find the percentage by mass of each element in a compound by
experiment. Using this information, it is then possible to find the
simplest formula of that compound. To do this we shall also need to know
the relative atomic mass of each element present in the compound.
work out the empirical formula you need to know the masses of elements
that combine. For example, magnesium combines with oxygen to form
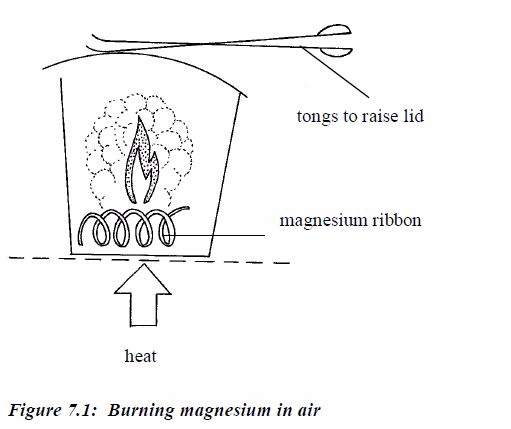
magnesium oxide. The masses that combine can be found like this:
- Weigh a crucible and lid, empty. Then add a coil of magnesium ribbon and weigh again.
- Heat the crucible. Raise the lid carefully at intervals to let oxygen in. The magnesium ribbon burns brightly.
- When
burning is complete, let the crucible cool (still with its lid on).
Then, weigh again. The increase in mass is due to oxygen.
- Mass of crucible + lid = 25.2 g
- Mass of crucible + lid + magnesium = 27.6g
- Mass of crucible + lid + magnesium oxide = 29.2g
- Mass of magnesium = 27.6g – 25.2g = 2.4g
- Mass of magnesium oxide = 29.2g – 25.2g = 4.0g
- Mass of oxygen, therefore = 4.0g – 2.4g =1.6g
2.4g of magnesium combines with 1.6g of oxygen.The RAMs are Mg = 24, O =
16. Changing masses to moles:24/2.4moles of magnesium atoms combines
with1.6/16 moles of oxygen atoms
moles magnesium combines with 0.1moles of oxygen atoms.So the atoms
combine in a ratio of 0.1:0.1 or simply 1:1The empirical formula of
magnesium oxide = MgO
the empirical formula can be determined from the provided percentage
composition or weight of atoms of the elements that constitute a
compound.
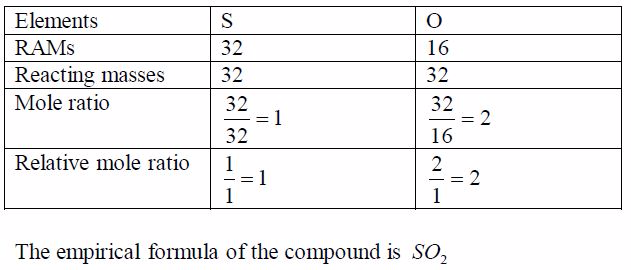
experiment shows that 32g of sulphur combines with 32g ofoxygen to form
the compound sulphur dioxide. What is itsempirical formula?
each mass by the RAM of the respective element. This gives the ratio of
different numbers of atoms of each element that make up the compound.
Again, divide each of these ratios by the smallest to get the whole
number ratio. This gives the simplest ratio of the constituent elements
possible. Sometimes you may not get a whole number ratio. If this
happens, round off the ratio to the nearest whole number. Finally, write
down the formula using the obtained ratio of the elements. Study the
following table and make sure you understand the procedure:
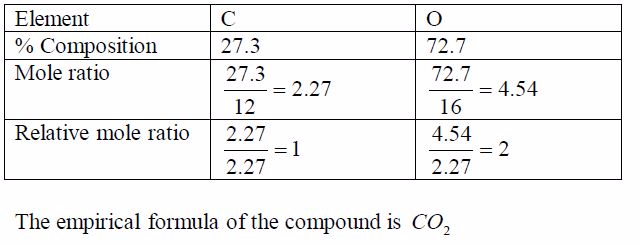
oxide of carbon contains 27.3% carbon. Find the empirical formula of
the oxide.Think big!What other element is present in the oxide of
carbon?How do you know that the percentage of this other element
is72.7%?
order to work out the simplest formula we divide each percentage by the
relative atomic mass of each element. This allows a comparison of the
different numbers of atoms of each element that are present. We get a
ratio of each element with respect to each other as worked out in the
table below. To get a whole number ratio, we again, divide each of these
ratios by the smallest.The result shows the simplest ratio of atoms, in
this case one carbon atom to two oxygen atoms. The simplest ratio,
therefore,is CO2 .
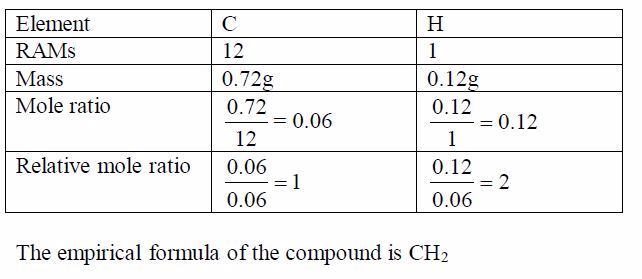
compound X is a hydrocarbon. It contains only carbon and hydrogen
atoms. 0.84g of X was completely burned in air. This produced 2.64g of
carbon dioxide CO2 and 1.08g of water H2 O. Find the empirical formula
of X.
- In CO2 ,12/44of the mass is carbon
- All the carbon came from X
2.64g of CO2 contains 2.64g〈12/44〉*2.64g= 0.72g of carbon
and,therefore, 0.12g of hydrogen (0.84g – 0.72g = 0.12g)Since we have
deduced the weights of the respective elements in the compound, we can
now work out the empirical formula as usual:
molecular formula is more useful than the empirical formula because it
gives more information. For some molecular compounds, both formulae are
the same. For others they are different.
- the empirical formula; and
- the molecular weight of the compound.
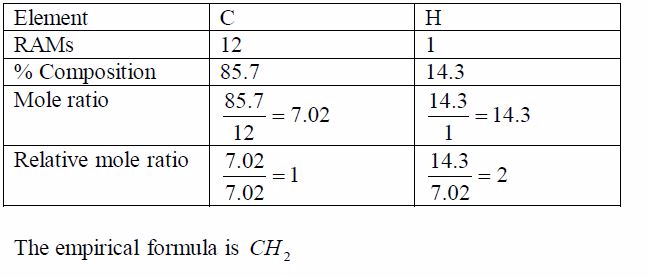
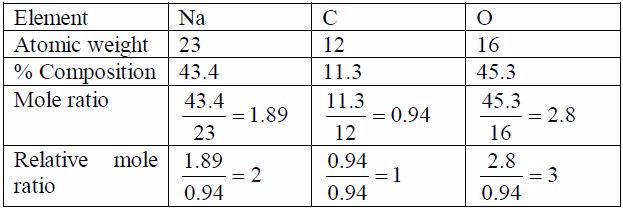
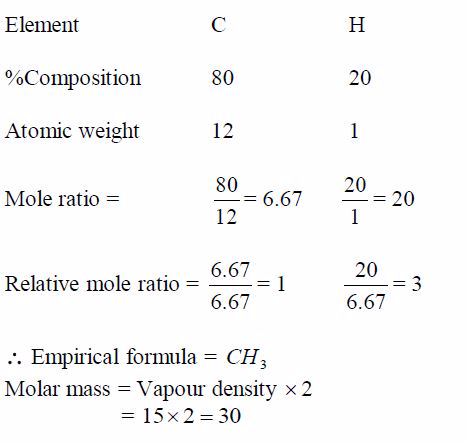
Example 5
Step 1: Find the empirical formula of the compound:
Step 2: Find the molecular formula using the relation 2 CH n =Molecular formula:

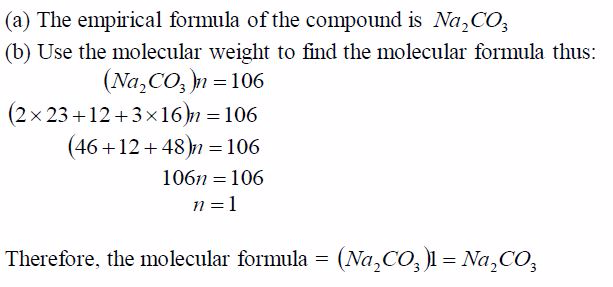
some cases, the molecular weights are given while in others the vapour
density is given. To get the molecular weight,multiply the vapour
density by two, e.g.:Molecular weight = Vapour density 2
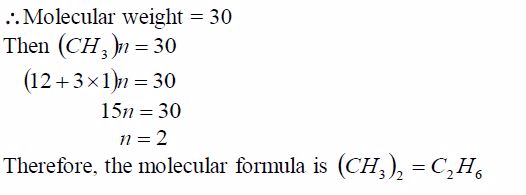
state (or oxidation number) gives information about the number of
electrons an element has lost, gained or shared on forming a compound.
An element gains, loses or shares electron(s) only when it reacts to
form a compound. An element in a free state has an oxidation state of
zero.
is a close correlation between valency and oxidation state.Oxidation
state of an atom in a compound is normally assigned relative to the
other elements in that particular compound. So you find that the
oxidation state of a particular atom may change depending on a compound
in which it is. For instance, the oxidation state of sulphur in SO2 is
+4, whereas in SO3 is +6.
2: Some elements nearly always have the same oxidation number in their
compounds. These elements can be used as reference points in assigning
oxidation numbers to the other elements. For example:
- Fluorine in all its compounds shows an oxidation number of -1.
- Chlorine in all its compounds has an oxidation number of -1 except when combined with fluorine or oxygen.
- Oxygen
in all its compounds has an oxidation state of -2except in peroxides
where it has an oxidation state of -1and oxygen difluoride (OF2) in
which it has an oxidation state of +2. - Hydrogen in all its
compounds exhibits an oxidation state of +1 except in metal hydroxides
where it shows an oxidation state of -1. - Potassium in all its compounds exhibits an oxidation state of +1.
3: The algebraic sum of oxidation numbers of all elementsin a radical
e.g. SO4 is equal to the charge possessed by theradical e.g. for the
sulphate radical ( SO4 ) it is -2.
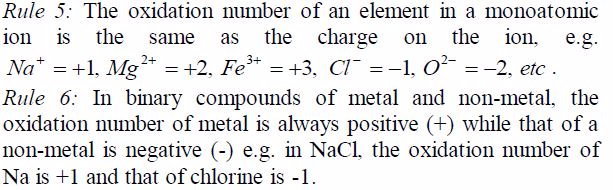
oxidation number of potassium, K = +1 and that of oxygen,O = —2. But
since there are 3 oxygen atoms, we have 2*3 =—6.KClO3 is a neutral
compound, so the sum of oxidation numbersof all elements in the compound
is zero.
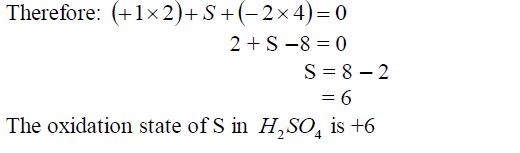
electrons are the electrons that participate in forming chemical bonds.
For example, lets look at the element carbon. Carbon has a total of 6
electrons (you can tell this by looking at the periodic table). However,
2 of those electrons are in the core of the atom ( in the 1s orbital).
The remaining 4 electrons are in the outer 2s and 2p orbitals. Since
these 4 electrons are in the outer shell, they can participate in
bonding. Therefore since there’s 4 electrons that carbon can share, we
say that carbon has a valency (or as you call it a valency number) of 4.
state is a number used to designate how oxidized an atom is in a
compound or molecule. It is the hypothetical charge an atom would have
if all of its bonds were completely ionic (rather than covalent). Really
oxidation state is a book-keeping formalism that allows us to track
what is being oxidized or reduced in a chemical reaction by comparing
the oxidation states of the reactants to those of the products.Hope that
helps.
Radicals
The Concept of Radicals
Explain the concept of radicals
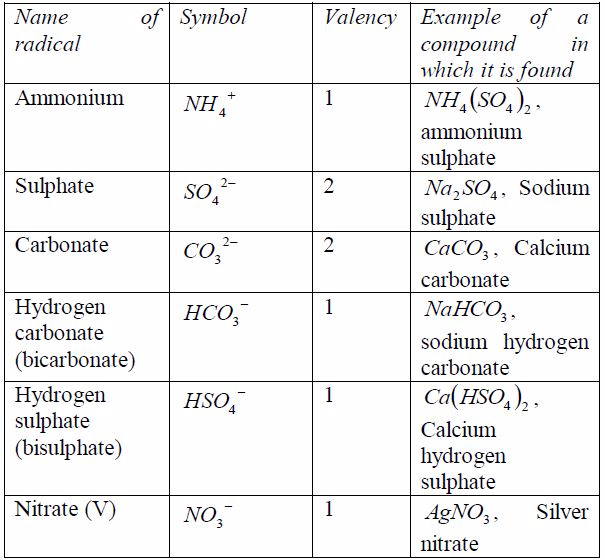
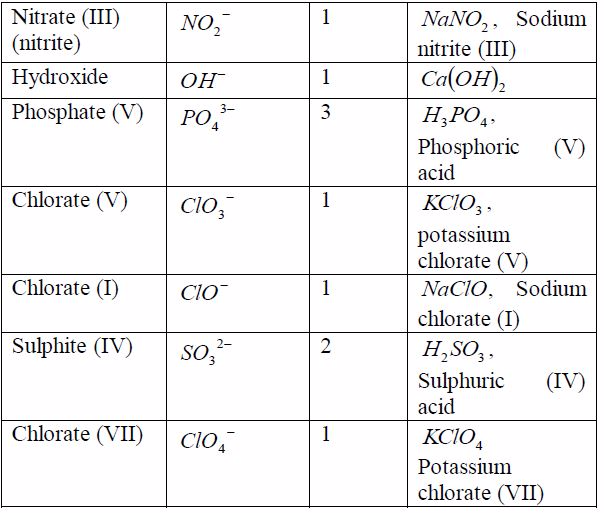
radical is a group of elements which behaves like a singleatom in
forming compounds. Radicals do not exist alone. Theyare always found
combined with metals. The valency of theradical is equal to the charge
on it. Table 7.6 shows the valenciesof different radicals. Examples of
compounds in which theradicals can be found are also included in the
table. All radicalsare charged. The only common positively charged
radical isammonium radical. All these radicals except ammonium
radical,which is positively charged, can combine with hydrogen or
othermetals.
Table 7.6: Some radicals and their valencies
As
you have seen in the examples in the table above and in theprevious
section, when writing the formula of any substance,you have to take into
account the valencies of the reactingelements or radicals.
1: Identify the elements or radicals in the compound:Sodium sulphate is
a compound whose every molecule is madeup of a sodium metal and a
sulphate radical.

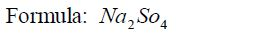
you have seen in the examples in the table above and in theprevious
section, when writing the formula of any substance,you have to take into
account the valencies of the reactingelements or radicals. the example
is above
bonding is a type of bonding which involves equalsharing of electrons
between two or more atoms participating inbond formation. It is,
generally, the property of non-metals to form covalent bonds. In a
normal covalent bond, only electronsin the outermost shell of an atom
are available for bondformation. Atoms share electrons so as to form a
stable electronstructure of the nearby noble gas atom. Consider the
bonding inthe following atoms:
atom possesses only one electron. Its shell holds amaximum of 2
electrons, so it is not full. When two hydrogenatoms make a bond, each
one donates one electron to the sharedpair to form a stable helium
structure.
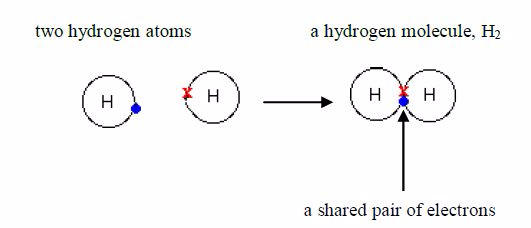
atoms share electrons, there is a strong force ofattraction between
them, holding them together. This force iscalled a covalent bond. The
bonded atoms form a molecule.
gas is made up of hydrogen molecules and, for thisreason; it is called a
molecular substance. Its formula is 2 HSeveral other non-metals are
also molecular. For exampleChlorine, Cl2 , nitrogen, N2 iodine, I2 ,
oxygen O2 , sulphur,S8 , phosphorus, P4 , etc.
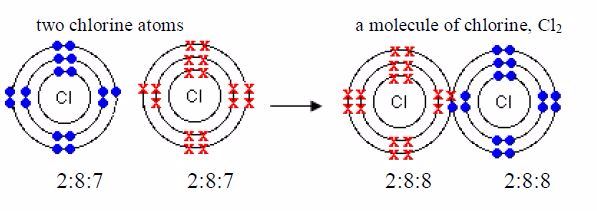
molecule of chlorine is made up of two chlorine atoms.Each chlorine
atom has an electronic configuration of 2:8:7. Itsconfiguration is only
one electron less than the stable structurea shared pair of electrons of
argon, 2,8,8. Each of the two chlorine atoms contributes oneelectron to
the shared pair during chemical combination.
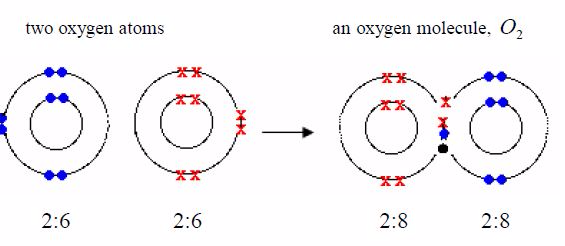
formula for oxygen is O2 . So each molecule must containtwo atoms. The
electronic structure of oxygen is 2:6. Eachoxygen atom has only six
outer electrons. So it needs two moreelectrons to reach a stable neon
structure, 2:8. Therefore, eachatom contributes two electrons to be
shared.
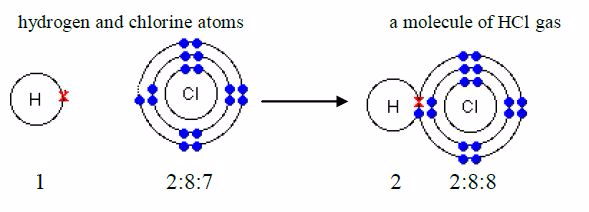
formula for hydrogen chloride is HCl. The electronicstructure for
hydrogen atom is 1 and that for chlorine atom is2:8:7. An atom of
hydrogen has 1 electron in its shell and achlorine atom has 7 outer
electrons. To form a stable dupletstructure of helium, 2, the outer
shell of hydrogen atom mustreceive one more electron and in order to
form a stable argon structure, 2:8:8, the outer shell of chlorine atom
must receiveone more electron. So, each atom must contribute one
electronfor sharing.
the above four examples, each hydrogen atom acquire thehelium structure
(duplet), each chlorine atom achieves the argonconfiguration 2:8:8
(octet) and each oxygen atom acquires theneon configuration, 2:8
(octet).
is possible to form multiple bonds between two non-metalatoms. When two
electrons are shared between two atoms, onefrom each atom, we represent
them by a single line, e.g. Cl-Cl.When four electrons are shared such
that each atom contributestwo electrons, we may represent the double
bond formed by twolines, e.g. O=O. Likewise, when six electrons are
shared suchthat each atom contributes three electrons, we may represent
thetriple bond by three lines, N≡N.
have seen that several non-metal elements exist asmolecules. A huge
number of compounds also exist asmolecules. In a molecular compound,
atoms of differentelements share electrons with each other. These
compounds areoften called electrovalent compounds because of the
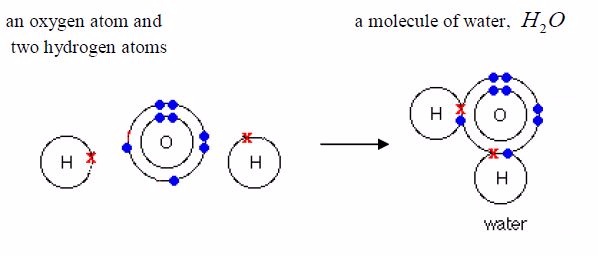
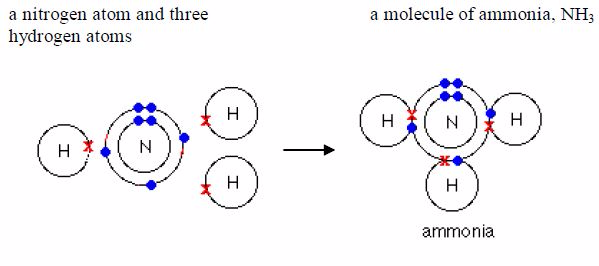
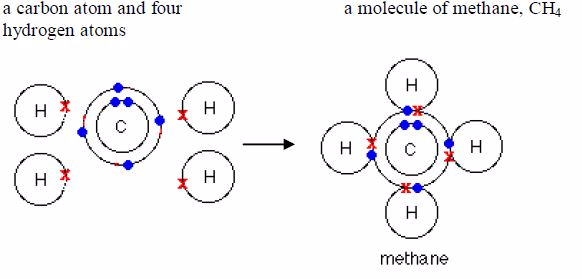
covalentbond in them. Water, ammonia and methane are examples.Remember
it was pointed early that only the electrons in theoutermost shells take
part in bonding. As you can see in the examples below, only electrons
in the outermost shells areinvolved in bond formation. Electrons in the
inner shells do notnormally take part in bonding.
- They are often liquids and gases at room temperature.
- They have low melting and boiling points (low heats of fusion and vapourization).
- They
are usually soluble in organic solvents such as ethanol, ether,
benzene, or carbon disulphide (very few are soluble in water). - They
do not conduct electricity because they contain no ions and so are
non-electrolytes. Electrovalent compounds consist of molecules.
Electrovalent Bonding
type of bonding occurs when an atom transfers electron(s) from its
outermost shell to the outermost shell of another atom, usually a
non-metal. In this type of bonding, an atom of a metallic element or
group loses its valency electrons from its outermost shell. The lost
electrons pass over to the outer shells of an atom with which the metal
is combining. By so doing, the metal will become positively charged due
to excess proton(s) left on the nucleus while the non-metal will become
negatively charged due to extra electron(s) it has received. The
particles are then known as ions. The positive ions are called cations
while the negative ions are called anions.
atoms lose electrons to attain the electron configuration ofthe nearest
noble gas, while the non-metal atoms gain electronsto attain the
electron configuration of the nearest noble gas. Thismeans that an
electron octet is left behind in the metal andcreated in the non-metal.
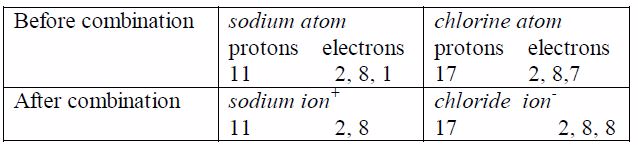
ions now posses stable outer octets, like a noble gas.As you have seen,
no molecule of sodium chloride is formed.Cations ( Na ) and anions Cl
attract one another and arrangethemselves into a rigid, solid shape
called a crystal, but theyremain quite separate. The combination can be
expressed only inionic form as NaCl
ionic compound is thus a cluster of ions in which a positiveion is
surrounded spatially by a number of negative ions, while anumber of
positive ions similarly surround each negative ion.The force holding
together the oppositely charged ions is calledelectrostatic force and
hence the name electrovalent bond.
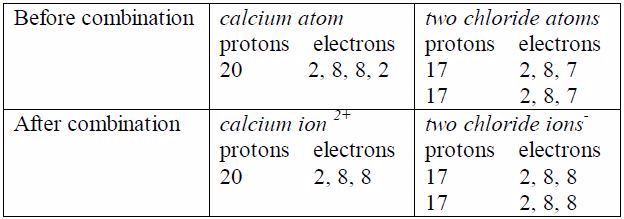
the calcium ion, the two excess nuclear protons produce adouble
positive charge, and the two electrons released from theouter shell of
calcium atom are equally shared between the twochloride atoms. In each
chloride ion, the excess electronproduces a single negative charge that
is Ca 2Cl
Activity 1
Magnesium
oxide is an electrovalent compound just like calcium oxide and sodium
chloride. Show how the magnesium atom combines with oxygen atom to form
the oxide, clearly indicating the process of electron loss and gain
Properties of electrovalent (ionic) compounds
- Ionic compounds are generally crystalline solids at room temperature.
- They have high melting and boiling points (also high heats of fusion and vapourization)
- They are generally soluble in water but insoluble in organic solvents such as benzene, alcohol and ether.
- They conduct electricity when molten or when dissolved in water (not when solid)



No comments:
Post a Comment