
The Concept of Hardness of Water
Explain the concept of hardness of water
It contains natural compounds dissolved from rocks and soil. It may also contain traces of chemicals dumped from homes, farms and factories.
One can distinguish between hard and soft water when washing with soap. Hard water does not form lather easily. Instead, it forms a precipitate or scum. It requires much soap to react with all the dissolved minerals before enough lather is formed. Therefore, hard water wastes soap during washing.
When soap is used with hard water a “scum” forms on the surface. This is a result of a precipitation reaction between calcium and/or magnesium ions and soap. Soaps are the sodium or potassium salts of long-chain organic acids.
Soaps are made from animal fats by treatment with alkali (NaOH or KOH). Ordinary washing soap is a compound of stearic acid, C17H35COOH. The nature of such soaps is the salt, sodium stearate, C17H35COONa+. Sodium stearate is soluble in water but calcium stearate is not.
When soap is mixed with hard water, the calcium or magnesium salts in the hard water react with soap and precipitates as scum. The nature of scum is either calcium stearate or magnesium stearate:
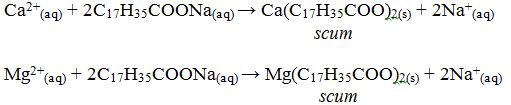
The problem of scum formation only occurs with soaps. Soapless detergents do not produce scum. The trade names for some soapy detergents sold in Tanzania include Komoa, Kuku, Taifa, Mbuni, Mshindi, Changu, Jamaa and several other bar soaps. The trade names for some soapless detergents include Omo, Foma, Tesa, Toss, Dynamo, Swan, etc.
Causes of Permanent and Temporary Hardness in Water
State causes of permanent and temporary hardness in water
Water is generally said to be hard if it contains soluble salts of calcium and magnesium. The salts are calcium and magnesium sulphates and hydrogencarbonates. Hardness of water is caused by higher than usual levels of calcium (Ca2+) and magnesium (Mg2+) ions in water.
As rain water passes through the atmosphere, it dissolves carbondioxide to form a weak carbonic acid.
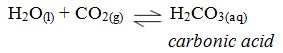
As this solution passes over and through rocks containing chalk, limestone or dolomite, the rainwater very slowly dissolves them:
H2CO3(aq) + CaCO3(s) → Ca(HCO3)2 (aq)
The calcium hydrogencarbonate formed is soluble in water and is responsible for the presence of calcium (Ca2+) ions in water.
Some of the rocks may contain gypsum (CaSO4.2H2O), anhydrite (CaSO4), Kieserite (MgSO4.H2O) or dolomite (CaCO3. MgCO3) which can dissolve to a limited extent in water. The presence of these dissolved substances also causes the water to be hard. These substances dissolve sparingly in water to form Ca2+ and Mg2+ ions which are responsible for water hardness as stated early.
Activity 1
Investigation of the causes of water hardness
Materials:
- Test tube rack-
- Five clean test tubes
- Measuring cylinder (100cm3)
- Calcium sulphate solution (1 mol dm-3)
- Soap solution
- Magnesium sulphate solution (1 mol dm-3)
- Sodium sulphate solution (1 mol dm-3)
- Potassium sulphate solution (1 mol dm-3)
- Distilled water
Procedure:
- Label five clean and dry test tubes as A, B, C, D and E. 2
- Add 10 cm3 of 1.0M calcium, magnesium, sodium and potassium sulphate solutions and distilled water in each of the test tubes respectively.
- Add 5 cm3 of soap in each test tube
- Shake the test tubes well and place them in a test tube rack
- Observe the amount of lather formed in each test tube, and if there is any precipitate (scum) formed.
Test tube |
Salt present |
Ions present in solution of salt |
Lather or scum formed? |
Water hard or soft? |
| A | calcium sulphate | Ca2+, SO42- | scum is formed | Hard |
| B | magnesium sulphate | Mg2+ , SO42- | scum is formed | hard |
| C | sodium sulphate | Na+, SO42- | lather is formed | soft |
| D | potassium sulphate | K+, SO42- | lather is formed | soft |
| E | distilled water | no ions | lather is formed | soft |
Interpretation of the results
From the result of experiment, we can conclude that scum is produced when either calcium or magnesium salt is present in water. So, high levels of calcium or magnesium ions in water are responsible for water hardness.
Types of Hardness of Water
Types of Hardness of Water
Identify types of hardness of water
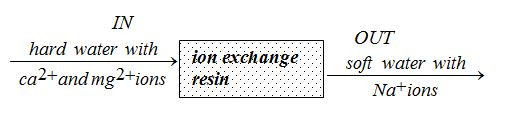
An ion exchange column removes ca2+ and mg2+ ions from the water and replaces them with Na+ ions
When all sodium ions have been removed from the resin, it is regenerated by pouring a concentrated solution of sodium chloride through it. The sodium ions remove the calcium and/or magnesium ions off the resin and the ion exchanger is ready for the use again.Other ions could also be used instead of sodium for the resin. But sodium chloride is normally used to supply the sodium ions because salt is cheap.
Use of softeners
Many modern washing powders now have softeners added to them. The softeners are often phosphates. The phosphates ions react with calcium ions to form calcium phosphate and remove the hardness.3Ca2+(aq)+ 2PO43-(aq)→ Ca3(PO4)2(s)
The Importance of Hard Water Treatment and Purification
Describe the importance of hard water treatment and purification
The significance of water in daily life is well known to everyone. The water we obtain from natural sources is never pure. It contains dissolved minerals which render the water unfit for direct uses.
The water from some sources contains calcium and magnesium compounds dissolved in it. These compounds are responsible for water hardness. To make the water fit for various uses, it is imperative to remove the hardness. The following points state why it is important to treat and purify hard water:
Hard water wastes soap. To get enough lather with hard water, it requires more soap than it does with soft water. So it is important to soften the water in order to save the soap and hence reduce the cost of washing. Laundry uses less soap and can be done at lower temperatures.
Treating and purifying hard water eliminates the possibility of forming limescale deposits in water boilers, kettles, washing machines, water heaters, shower heads and dish washers. The scale formed around the heating elements can cause the element to overheat and fail
Treated and purified water leaves no scum on clothes during washing. Scum spoils the finishing of some fabrics. It forms nasty deposits (marks) on clothing that has been washed.
Softened water has the advantage of not blocking the water pipes. In industry, deposits of scales can block the pipes in boilers. This is a safety hazard as it could cause pressure to build up until there is an explosion. A similar coating can occur in hot water pipes at home and in central heating systems.
The Importance of Hard Water in Daily Life
Hard water is not always disadvantageous. The following points explain the importance of hard water:
<> The dissolved calcium and magnesium salts improve the taste of water. Distilled water is tasteless and quite unpleasant to drink. This is why water-processing plants add some salts in the distilled water to make it tasteful.
<> Calcium dissolved in hard water is an essential mineral for growth of bones and teeth. It makes our teeth and bones hard, strong and resistant to shear and pressure.
<> In some places, old lead pipes are used for water supply. Lead is very poisonous, and a little of it can dissolve in soft water. But the carbonate (CO32-) or sulphate (SO42-) ions present in hard water reacts with lead to form a coating of lead carbonate or lead sulphate that prevents lead from dissolving. This prevents lead poisoning.
<> A coating of calcium carbonate inside pipes, boilers and radiators helps to prevent corrosion.
<> In recent years, it has been suggested that drinking hard water helps to prevent heart diseases. 6. It has also been found that hard water is good for brewing beer.


No comments:
Post a Comment